Part B
Identifying & Interpreting Sedimentary Rocks
Sedimentary rocks can form from the depsotion of loose sediment of various sizes derived from preexisting rock (like mud, sand, and gravel), from mineral crystals (like silica or calcite) that evaporate or precipitate from water, or from the accumulation of organic material. Over a relatively long period of time, the sediment undergoes lithification (literally, turned to stone), which may involve compaction, cementation, evaporation, and/or precipitation. From these processes, sedimentary rocks are formed (see Figure 7-9).
As with the igneous rocks, field identification and interpretation of sedimentary rocks requires knowledge of both the texture and composition of the rock. In classifying a sedimentary rock, you first determine its texture: is it made of pieces of pre-existing minerals or rocks, or is it made of shells or interlocking crystals that grew together? Clastic sedimentary rocks are made of pieces (also called grains or clasts) and are cemented together with calcite, quartz, clay, etc. For non-clastic or chemical rocks, you need to determine the composition of the shells or mineral crystals in the rock. These rocks include chemical sedimentary rocks usually formed from the precipitation or evaporation of water at/near the Earth's surface. Biochemical sedimentary rocks form as a result of biological activity and typically consist of inorganic shell material of various sizes. Organic sedimentary rocks form largely from the accumulated remains of organic material.
|
Figure 7-9. The most common sedimentary rocks have either clastic and chemical texture. Clastic rocks form the flatirons of the Rocky Mountains' Front Range in Boulder, Colorado (left). |
|
Clastic Rock Texture
Clastic sedimentary rocks are ones made of sediment or broken pieces (clasts or fragments) of other rock (see Figure 7-10). Clasts can range in size (fine to coarse), shape (rounded or angular), and sorting (variation in grain size). If you think a rock has this texture, then you can describe it just like clastic sediment.
Clastic Texture Properties
If you think a rock has clastic texture, then you can describe it just like clastic sediment:
Clast size
> Are the clasts gravel-sized (>2 mm), sand-sized (2 mm to 1/16 mm), or mud-sized (<1/16 mm)? If it's really hard to see individual clasts, then it's probably mud-sized (silt or clay).
Clast rounding
> Are the clasts mostly rounded or angular?
Clast sorting
> Are the clasts mostly the same size (well sorted) or different sizes (poorly sorted)?
IMPORTANT NOTES
> Being able to distinguish between a fine-grained clastic rock and a fine-grained crystalline rock is not always easy because the grains or crystals are so small. You'll need some other clues in this case.
> To accurately name the rock, look at a representative part of the rock. Look at the size, rounding, and sorting of the majority of clasts in a rock, not just a few.
> The particles in a clastic rock are typically cemented together by minerals deposited by groundwater processes (during burial and lithification). Common cements include silica (especially in silica-rich sandstones), calcite, iron oxide, etc. The degree of cementation can vary.
> Clastic rocks with significant amounts of calcite clasts or calcite cement will react with HCl acid, just like the chemical-textured carbonate rocks do. The HCl reaction doesn't relate to the rock texture, but onlyto the presence of calcite in the rock.
Chemical Rock Texture
Not all sedimentary rocks are made of pieces. Chemical sedimentary rocks commonly have a crystalline appearance (see Figure 7-11) and are classified based on their mineral composition.
|
Figure 7-11. Chemical (crystalline) texture in a sedimentary rock with chemical (crystalline) texture. |
Chemical Texture Properties & Minerals
If you think a rock has chemical texture, then you need to use a combination of physical properties (hardness, HCl acid test, color, etc.) to identify the mineral composition of the rock or shells. Some of the diagnostic properties include:
Effervescence (reaction with HCl acid)
> The hydrochloric (HCl) acid test is used to identify the presence or absence of carbonate minerals like calcite and calcite-rich rocks.
> A few drops of the dilute HCl acid applied to carbonate minerals (like calcite) and calcite-rich rocks will produce an effervescent ("fizzing" or bubbling) reaction:
CaCO3 (calcite) + 2HCl (hydrochloric acid) >> CaCl2 + H2O + CO2 (carbon dioxide "fizz")
> WARNING - A few drops of dilute hydrochloric acid won't harm you if you get it on your fingers; just wash it off. However, it may stain clothing or furniture, and you certainly don't want to get it in your eyes or mouth.
|
Figure 7-12. Performing the effervescence test on a rock in the field. Joann trickles dilute hydrochloric acid (HCl) on a rock sample. If it "fizzes", it's a carbonate rock. |
|
Hardness
> Some minerals are more resistant to scratching than others, and can be compared using a relative scale of hardness (Mohs Scale - the softer the mineral, the lower its relative hardness number, H).
> The hardness test involves scratching a mineral/rock with a steel nail, your fingernail, etc. to determine its relative hardness. Use materials with known relative hardness, like your fingernail (H = 2.5) or a steel nail (H = 5.5). If your fingernail scratches the mineral, then the mineral is relatively soft (H < 2.5). If a steel nail cannot scratch the mineral, then the mineral is relatively hard (H > 5.5).
Shape
> Mineral crystals can form characteristic shapes as they grow (crystal habit). For example, quartz crystals have six-sided hexagonal shapes..
> Minerals can also break into characteristic shapes along certain planes (cleavage) or split in random directions (fracture). For example, calcite cleaves into angled rhombohedra, whereas quartz fractures randomly.
Color
> Color is usually the most noticeable property, but since most minerals come in a variety of colors, it can also be the least diagnostic. For instance, quartz can occur in many different colors due to minute impurities (rose quartz = pink, smoky quartz = black, amethyst = purple), even though they all have the same basic composition.
Some of the common minerals present in sedimentary rocks include:
Silica / SiO2 / Very hard (H = 7), so it cannot be scratched by a steel nail; fractures along irregular surfaces; will not "fizz" with HCl acid; glassy, variable color. This is a common cement between grains in a clastic rock.
Calcite / CaCO3 / Relatively soft (H = 3), so it can be scratched with a steel nail, but not with a fingernail; can break into rhombic shapes; reacts with HCl acid; variable color. This is a common cement between grains in a clastic rock.
Dolomite / (Ca,Mg)(CaCO3)2 / Essentially the same as calcite, except that it will not react with HCl acid as easily (need to be powdered).
Gypsum / CaSO4 * 2H2O / Very soft (H = 2), so it can be scratched with a fingernail; tabular shape or fibrous; will not "fizz" with HCl acid; typically clear to white in color. This is a common evaporite mineral.
Halite / NaCl / Relatively soft (H = 2.5), so it can be scratched with a steel nail, but not as easily with a fingernail; forms and breaks into cubic-shaped crystals; usually clear, white, or pink; tastes salty (but please, don't lick it!). This is a common evaporite mineral.
Hematite / Fe2O3 / Soft, so it can be scratched with a steel nail, but not with a fingernail; commonly brick red or silver in color; leaves a rusty-red streak; tarnishes to brownish-red.
IMPORTANT NOTES
> Any rock can "fizz" with HCl acid if it has any calcite in it or on it. Make sure you apply the acid to a fresh, representative surface because calcite is a common surficial mineral deposited by groundwater. Also, clastic rocks (like sandstone, conglomerate, etc.) can "fizz" with HCl acid if their clasts are made of calcite or if the cement binding the clasts together is composed of calcite (which is common).
> As the amount of dolomite and calcite in a carbonate rock can be variable, so can the degree of "fizzing". Because of the different relative proportions of the two minerals, some limestones will react vigorously, others less so. The same is true for dolostones, where some will not react at all and some will weakly react. The "tweener" rocks with the intermediate reactions may be called dolomitic limestones or limey dolostones.
Common Sedimentary Rocks
> A clastic sedimentary rock made mostly of angular, gravel-sized (>2 mm) sediment. Typically poorly sorted.
> May or may not react with HCl acid, depending on the composition of the clasts and cement.
> Common depositional environments include high-energy areas like mountain streams, alluvial fans, glaciers, and reefs.
> Arizona examples - Camels Head Formation (Camelback Mountain and Papago Park areas in Phoenix), many modern debris flow and stream deposits.
> A clastic sedimentary rock mostly made of rounded, gravel-sized sediment. Typically poorly sorted, but not always.
> May or may not react with HCl acid, depending on the composition of the clasts and cement.
> Common depositional environments include high-energy areas like stream channels, alluvial fans, and beaches.
> Arizona examples - Shinarump Conglomerate (Painted Desert), Barnes Conglomerate (Sierra Ancha), Hotauta Conglomerate (Grand Canyon), Tertiary "basin-fill" in central and southern Arizona, and many modern stream deposits.
> A clastic sedimentary rock mostly made of sand-sized (2 mm to 1/16 mm) clasts.
> There are several compositional types of sandstone. For example, quartz arenite is composed of mostly quartz grains (>90%), whereas feldspathic arenite (arkose) has more feldspar, and wacke has >15% clay-sized material.
> Sorting and rounding may vary, and can show well-defined layering (bedding).
> Because of their sand-sized clasts, sandstones may feel "gritty" or like sandpaper. They may or may not react with HCl acid, depending on the composition of the clasts and cement. Although commonly composed of a lot of highly resistant silica, sandstone is typically scratched with a steel nail.
> Sedimentary structures may include sandstone commonly includes cross bedding, graded bedding, and ripple marks.
> Common depositional environments include stream channels, flood plains, river deltas, desert sand dunes, beaches, etc.
> Arizona examples - Tapeats Sandstone, Coconino Sandstone, Navajo Sandstone, etc. These units form many buttes and cliffs in northern Arizona.
> A clastic sedimentary rock composed of >50% mud-sized (< 1/16 mm) particles from silt (1/16 mm to 1/256 mm) to clay (< 1/256 mm).
> Mudstone composed of clay-sized grains and breaks apart in layers is called shale or claystone ; if the particles are silt-sized, the rock is called siltstone.
> Mudstone may or may not react with HCl acid, depending on the composition of the cement. It is also typically scratched with a steel nail.
> Sedimentary structures commonly include dessication cracks (mud cracks) and ripple marks.
> Common depositional environments include low-energy areas like flood plains, river deltas, playas, lakes, beaches, shallow marine selves, reefs, and deep marine basins.
> Arizona examples- Hermit Formation, Bright Angel Shale, Hakatai Shale (Grand Canyon) and Chinle Formation (Painted Desert), Mancos Shale, etc. These are noteworthy slope-forming units in northern Arizona.
> A carbonate rock that can be chemical (precipitation of calcite crystals) or biochemical (composed of animal skeletons or shells) in origin.
> Many different varieties mainly composed of calcite, so reacts with HCl acid and can be scratched with a steel nail.
> Many different varieties of biochemical limestone, including many types of fossils (usually marine in origin). Travertine is commonly banded. Tufa is porous.
> Common depositional environments include lakes and marine environments. Travertine and tufa are limestones deposited in caves or springs.
> Arizona examples - Redwall Limestone and Muav Limestone (Grand Canyon), Escabrosa Limestone (southeastern Arizona). These are commonly cliff-forming units.
Dolostone(sometimes called dolomite)![]()
> A carbonate rock very similar to limestone, but is composed mainly of the mineral dolomite.
> Like dolomite, dolostone will not react with HCl acid (unless powdered), but like limestone, dolostone can be scratched with a steel nail.
> Typically forms as Mg-rich brines (very salty water) replace the calcium in limestone of arid lakes and playas (dried-up lakes), lagoons, sabkhas (coastal salt flats), and shallow marine environments.
> Common depositional environments include arid lakes and playas (dried-up desert lakes), lagoons, sabkhas (coastal salt flats), and shallow marine environments.
> Arizona examples - Martin Formation (central Arizona), Temple Butte Formation (Grand Canyon), etc. Anywhere carbonates rocks are exposed, dolostone is also commonly present.
> Biochemical carbonate rocks having weakly cemented shells and/or shell fragments.
> Coquina has large (>2 mm), angular, and poorly sorted shells; chalk is made of microscopic shells (<1/16 mm).
> Common depositional environments include beaches, reefs, and shallow marine environments.
> Arizona examples - Coquina is not common is Arizona. Chalk may be locally present in some Tertiary rock units (Chalk Canyon Formation).
> A chemical or biochemical sedimentary rock mainly composed of microcrystalline silica (quartz).
> Chert does not react with HCl acid. It is very hard (H = 7) and cannot be scratched with a steel nail.
> Chert can be chemical (precipitation of silica crystals) or biochemical (composed of microscopic animal skeletons or shells) in origin.
> Chert can form from groundwater processes or in deep marine environments.
> Arizona examples - Chert beds and nodules are typically abundant in marine carbonate units (like the Kaibab Formation and Redwall Limestone of northern Arizona).
> A general term for chemical sedimentary rocks that form from the evaporation of water.
> There are many different kinds of evaporites, including rock gypsum (gypstone) is composed of gypsum, whereas rock salt is typically composed of halite.
> Neither rock gypsum nor rock salt will react with HCl acid. Both can be scratched with a steel nail. Rock gypsum (H = 2) can be scratched with your fingernail, whereas rock salt (H = 3) is harder to scratch with your fingernail, if at all. Gypsum crystals have distinctive cleavage and halite crystals form cubes when free-growing.
> Common depositional environments include playas (dried-up lakebed), river flood plains, beaches, sabkhas (arid coastal mud flats), etc.
> Arizona examples - The Luke Salt (in the subsurface of the western Phoenix Basin), various playas across Arizona (Willcox, Red Lake), and within several rock formations in northern Arizona (Toroweap Formation, Kaibab Formation, Moenkopi Formation, etc.).
> An organic sedimentary rock that forms from the accumulation of dead organic material.
> Coal is typically brittle and brown to black in color.
> Common depositional environments include swamps and marshes.
> Arizona examples - The Cretaceous Mesa Verde Group (Black Mesa, northeastern Arizona) has several coal-bearing units.
Field Identification
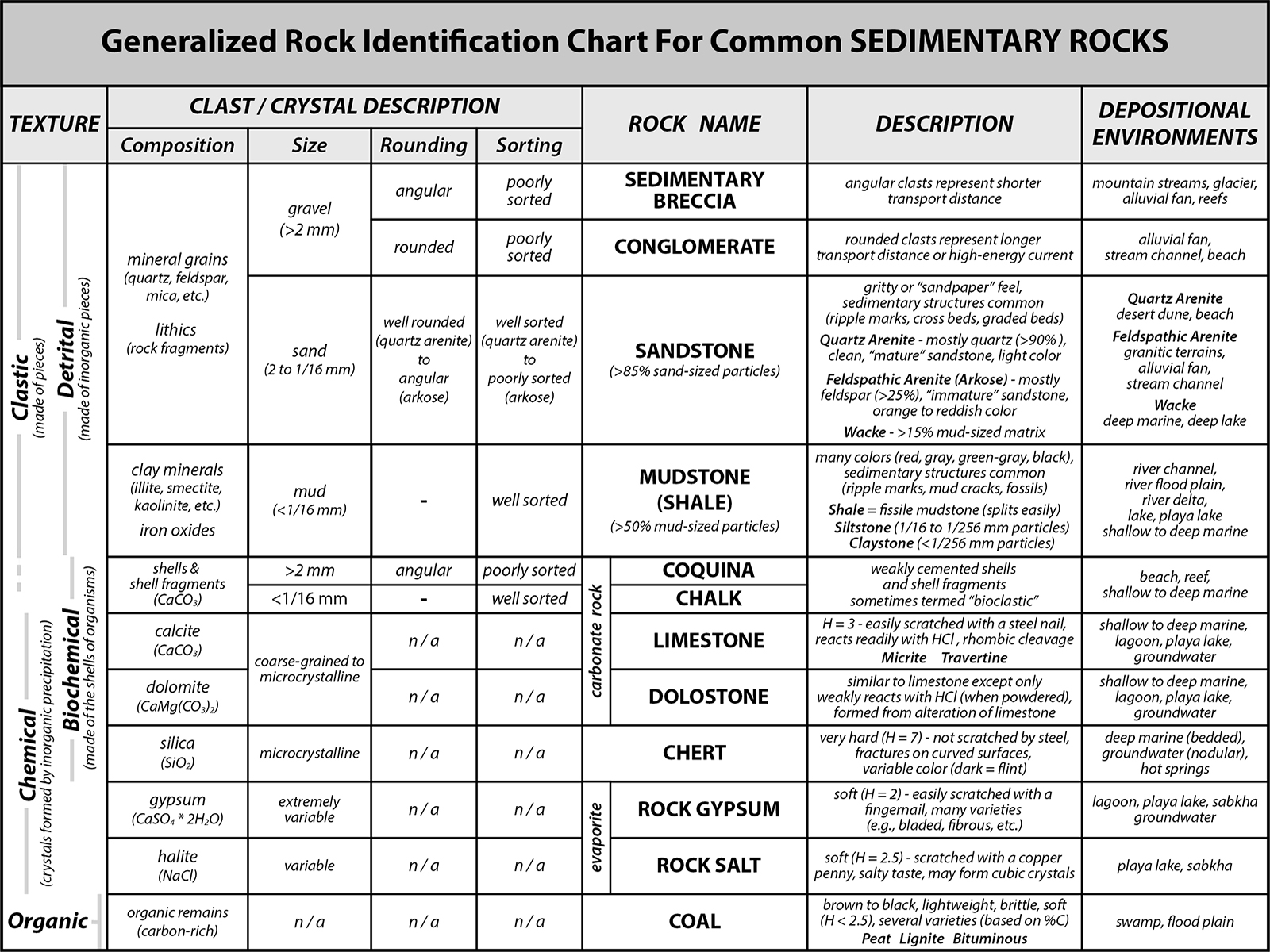
In classifying a sedimentary rock, you first look at its appearance (texture): is it made of pieces (clastic) or is it made of interlocking crystals that grow together (crystalline)? If clastic, then determine the size, rounding, sorting, and composition (if possible) of the clasts. If crystalline, determine the composition of the crystals. Then refer to the classification chart to name the rock (see Figure 7-13).k.
|
Figure 7-13. A rock identification chart for common sedimentary rocks. |
* IMPORTANT *
Using the PDF link above, print a hard-copy version of this chart for use in this lab and the lab quiz.
|
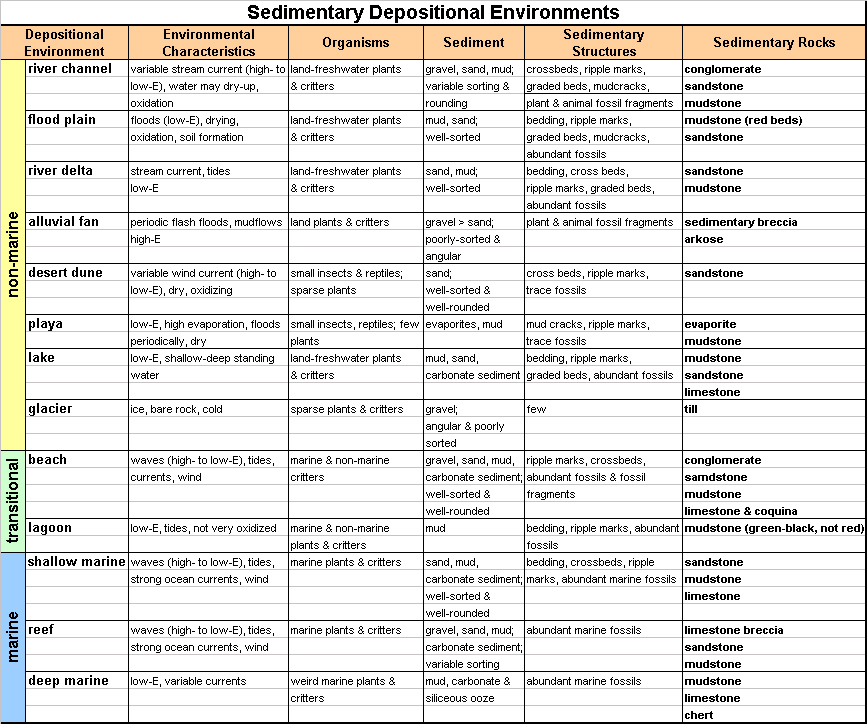
|
|
Figure 7-14. Depositional environment chart. Click HERE for a printable PDF version. |
For Quiz Me! questions B21 through B35, refer to the Sedimentary Rock Identification Chart (Figure 7-13), the Depositional Environment Chart (Figure 7-14), and the rock descriptions.
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()