Part B
Mineral Characteristics
What is a mineral, you ask? Well, a mineral is a naturally-occurring, inorganic substance with crystalline structure, a definite chemical composition, and diagnostic physical properties. So, what does that mean?
Naturally-occurring - You can find it in nature. Not man-made or synthetic. It should be noted that materials having characteristics very similar to those of real minerals can be manufactured in factories, but are considered synthetic minerals and are not true minerals in a geologic sense.
Inorganic - Not alive or composed of organic (carbon-based) matter. Almost all minerals are inorganic except for the rare instances where geological processes can form organic substances that can be considered minerals.
Crystalline structure - An orderly arrangement of atoms in a substance. Materials having crystalline structure can be called crystals.
Definite chemical composition - The same combination of elements (e.g., galena is always PbS, not Pb2S or PbS2. Slight variations can occur with secondary elements, but the main composition is always the same.
Diagnostic physical properties - A diverse suite of characteristics displayed by a substance (e.g., color, shape, hardness, etc.) that can be reliably used to identify the mineral.
|
Figure 4-3. Examples of minerals and non-minerals: solid water ice (a mineral), liquid water (not a mineral - no crystalline structure), pyrite (a mineral), and plastic (not a mineral - man-made, no crystalline structure, organic). |
|
Physical Properties of Minerals
Physical properties are the basic tools that you can use to discriminate one type of mineral from another. Let's learn about some of the important physical characteristics displayed by minerals. Once you are comfortable recognizing the various physical properties, you can then use them to identify some common minerals. A range of tools (magnifying glass, porcelain streak plate, steel nail, dilute HCl acid, magnet, etc.) are typically used to investigate the physical properties of minerals.
Specific Gravity
Specific Gravity is the ratio of the weight of a mineral to the weight of an equal volume of water. For example, water has a specific gravity (SG) of 1. Quartz has a SG of 2.65, meaning that quartz is 2.65 times heavier than an equal volume of water. The easiest way to determine the specific gravity of a mineral is to compare two minerals of equal size and judge how "heavy" the minerals feel. The mineral that is heavier has a higher specific gravity. Most rock-forming minerals have relatively low specific gravity, ranging between 2.6 and 3.5 (e.g., alkali feldspar ~ 2.6). Many metallic, ore-bearing minerals minerals tend to have higher specific gravity, ranging from 5 to 8 (e.g., pyrite ~5).
![]()
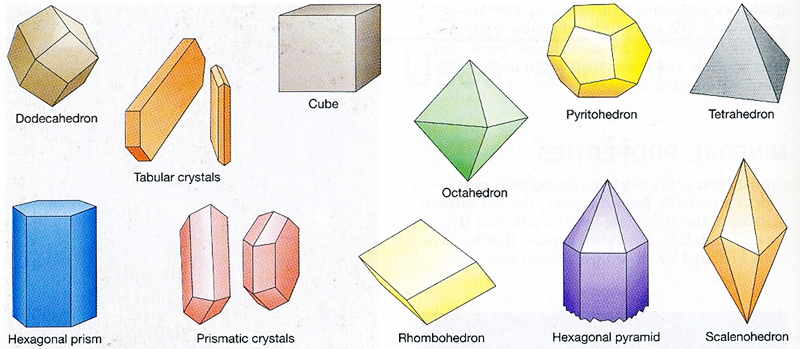
Crystal Habit
Crystal habit is the external, geometric appearance of a perfectly-formed crystal (see Figure 4-4). Some minerals have distinctive shapes, having many flat sides or crystal faces (e.g., well-formed quartz crystals have six sides, halite crystals form cubes, etc.) that are controlled by the atomic structure of the mineral. Minerals may form these crystalline shapes if allowed to grow freely. However, some minerals do not display any external crystal form at all.
|
|
|
Figure 4-4. There are many different types of crystal habit (from Boger et al., 1993). |
Refer to the various types of crystal habit in Figure 4-4 to answer Quiz Me! questions B05 and B06.
![]()
![]()
Color
Color is usually the most noticeable physical property. Minerals have the color of the light leaving their services which normally depends on: 1) the type of illuminating light, which may be ultraviolet, white light, infrared, etc., and 2) the reflectance properties of the mineral, which are largely dependent on composition and crystal structure of the mineral. Minerals may be colorless or have distinctive color (e.g., biotite is always black), and the economic minerals are typically colored (e.g., malachite is green). Some minerals display a range of colors, often do to small amounts of impurities within the mineral. For instance, quartz can occur as a clear mineral, but also as milky quartz, rose quartz, amethyst, smokey quartz, etc.), even though they all have the same basic composition (SiO2). Thus, it can also be the least diagnostic physical property.
|
|
|
Figure 4-5. Minerals can have many colors. Quartz is notorious for having different-colored forms. Thus, color may be distinctive, but is not always a reliable property when identifying minerals. |
Streak
Streak is the color of a mineral in powdered form. To determine streak, a mineral is scratched against a small, unglazed porcelain plate (streak plates). Some minerals leave a dark-colored streak; some minerals leave no streak at all.
|
|
|
Figure 4-6. Metallic minerals typically have a colored streak. |
Luster
Luster is a measure of how light interacts with the surface of a mineral. There are two basic types of luster: metallic and non-metallic. Metallic luster is produced by strong reflections from the mineral surface, making it look shiny like a metal on a fresh surface. These minerals can tarnish to a different color. Many of the sulfide ore minerals display metallic luster. There are many nonmetallic varieties of luster, ranging from the vitreous (or glassy) to resinous, greasy, earthy, etc. Most rock-forming minerals have non-metallic luster.
|
|
|
Figure 4-7. Different types of mineral luster. |
Refer to the various types of mineral luster in Figure 4-7 to answer Quiz Me! questions B07 and B08.
![]()
![]()
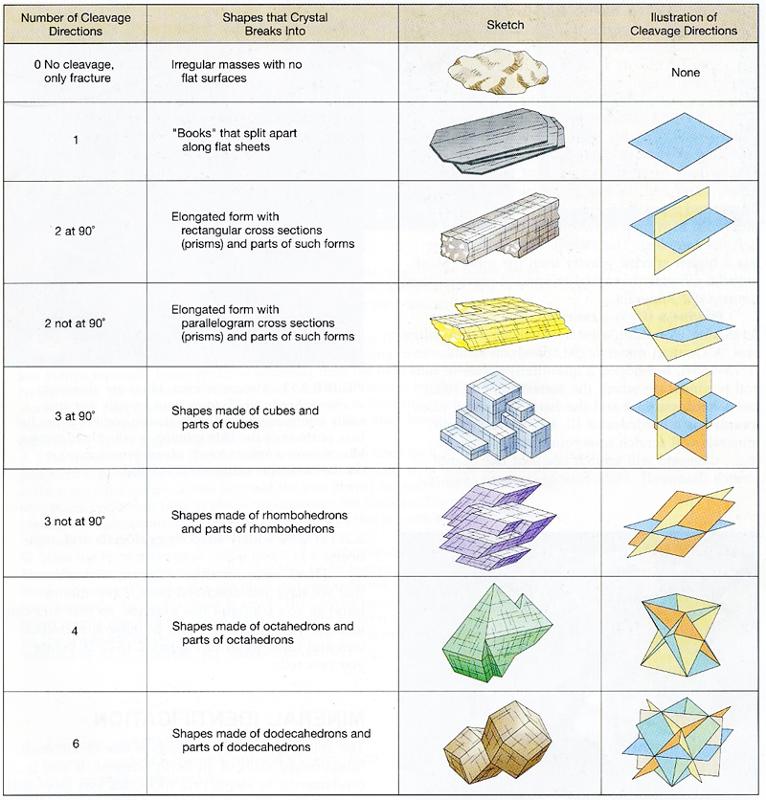
Cleavage
Cleavage is the tendency of a mineral to split ("cleave") along definite planes of weakness formed by its crystalline structure. Minerals may have one or more of these planes of weakness or cleavage planes, and when more than one is present we typically note number planes and the angle of their intersection.
> One plane of cleavage (basal cleavage) - The mica minerals, like muscovite and biotite, typically form "books" that break into sheets.
> Two planes of cleavage that intersect at right angles (90°) - Minerals like pyroxene and the feldspars have step-like corners formed by the two intersecting cleavage planes.
> Two planes of cleavage that intersect not at right angles (60° or 120°) - Gypsum and amphibole (hornblende) both have this type of cleavage.
> Three planes of cleavage that intersect at right angles (90°) (cubic cleavage) - Halite and galena form cubes along the three cleavage planes.
> Three planes of cleavage that intersect not at right angles (60° or 120°) (rhombic) - Calcite has this type of cleavage, which is like a slanted form of cubic cleavage.
> Four planes, six planes, etc. Yikes!
Fracture - Some minerals will not have any preferred directions of weakness and will break in random directions. There are different types of fracture, including hackly, irregular, and conchoidal. Quartz and volcanic glass both can break along a smoothly curving pattern called conchoidal fracture.
|
|
|
Figure 4-8. Different types of cleavage and fracture. |
![]()
![]()
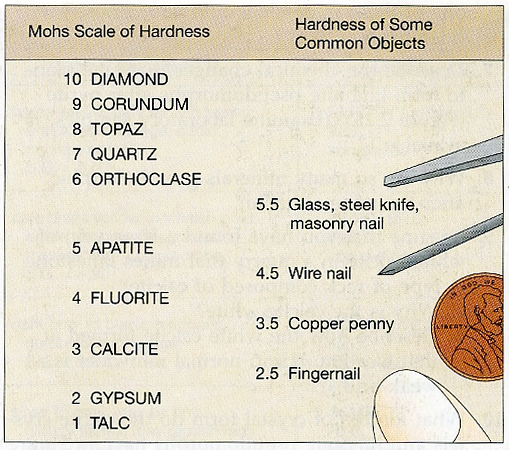
Hardness
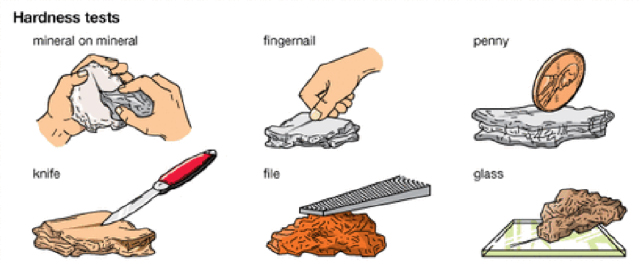
Hardness describe's a mineral's resistance to being scratched. Some minerals are more resistant to scratching than others, and can be compared using a relative scale of hardness called Mohs Scale (developed by German mineralogist Fredrich Mohs in the early 1800's). Basically, the softer the mineral, the lower its relative hardness number, H (see Figure 4-6). To determine the relative hardness of a mineral, use either your fingernail (H = 2.5), copper penny (H = 3.5), or a steel nail (H = 5.5). If your fingernail scratches the mineral, then the mineral is relatively soft (H < 2.5). If a steel nail cannot scratch the mineral, then the mineral is relatively hard (H > 5.5).
|
|
|
Figure 4-9a. Mohs scale of hardness and the relative hardness values of various common objects (from Boger et al., 1993). |
|
|
|
Figure 4-9b. We can easily test the hardness of a mineral by rubbing them with materials of known hardness (like a fingernail, penny, steel knife, plate of glass) and then observe which material was scratched, thus softer. |
![]()
![]()
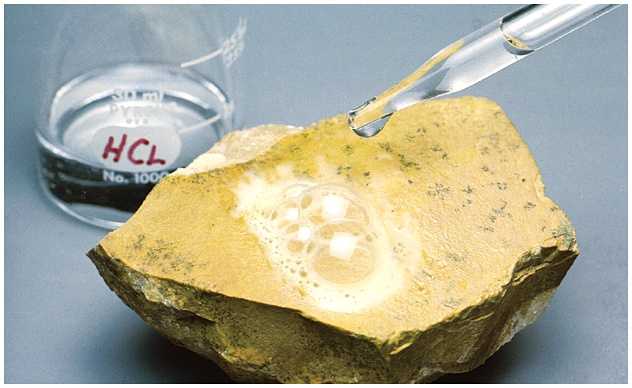
Efferevescence
Effervescence is the release of gas from a mineral as it reacts with an acid. Carbonate (CO3) minerals will typically react (effervesce, fizz, or bubble) with dilute HCl (hydrochloric) acid. This is a powerful test for the presence of calcite as fresh calcite will typically react vigorously, whereas dolomite will only react if it is powdered. Common sense precautions are necessary when using dilute HCl acid (i.e., wash your hands, don't drink it, etc.), although it is typically weak enough (1-3% HCl) that it is not a safety hazard if used responsibly. Undiluted vinegar may also work if dilute HCl acid is not available.
|
|
|
Figure 4-10. The mineral calcite will effervesce as carbon dioxide gas is generated during the reaction with dilute hydrochloric acid. |
Fluorescence
Florescence is the emission of light by a mineral after absorbing ultraviolet light. Some varieties of the minerals calcite and willemite can display strong florescence. Very cool!
|
|
|
Figure 4-11. The minerals calcite and willemite will fluoresce under UV light. |
Magnetism
A few minerals are naturally magnetic or are strongly attracted to a magnetic field or even a magnet. If you see a mineral sample with metallic objects sticking to it, it's probably magnetite, an iron ore that is the most magnetic mineral known.
|
|
|
Figure 4-12. Magnetite doing what magnetite does. |
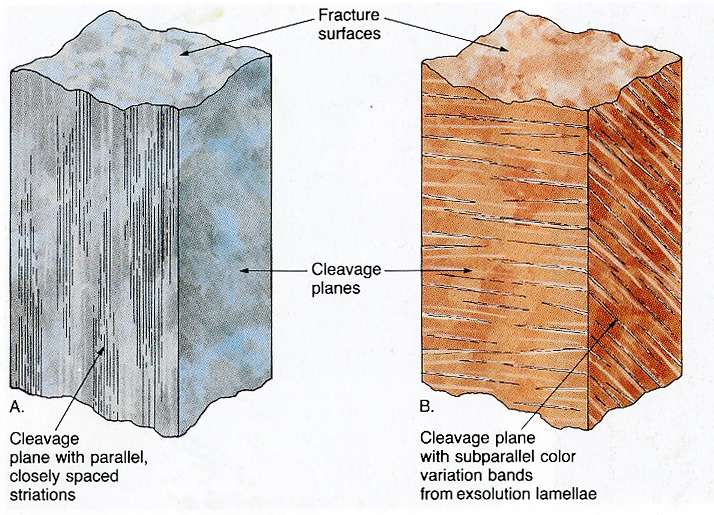
Striations & Exsolution Lamellae
Striations are crystal growth patterns or crystal habits that may form distinctive, fine, parallel grooves or striations on the cleavage planes of certain minerals. Plagioclase feldspar has these "hairline" grooves, which help distinguish it from the alkali feldspars. Pyrite also has distinctive striations. Alkali feldspar does not display striations, but does show exsolution lamellae, which are thin Na-rich intergrowths within the K-rich feldspar. Plagioclase feldspar does not display exsolution lamellae.
|
|
|
Figure 4-13a. Striations and exsolution lamellae (from Boger et al., 1993). |
|
|
|
Figure 4-13b. Striations in plagioclase feldspar. |
|
|
|
Figure 4-13c. Exsolution lamellae in alkali feldspar. |
Double Refraction
Double refraction results when the crystalline structure of a few minerals bends (refracts) light in such a way that two images are formed. Some types of clear calcite will display double refraction.
|
|
|
Figure 4-14. One variety of calcite, known as optical calcite or Iceland spar, has the unique property of double refraction. |
Quiz Me! questions B13 to B20 refer to the various mineral physical properties described above.
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()