Part A
It's Elemental
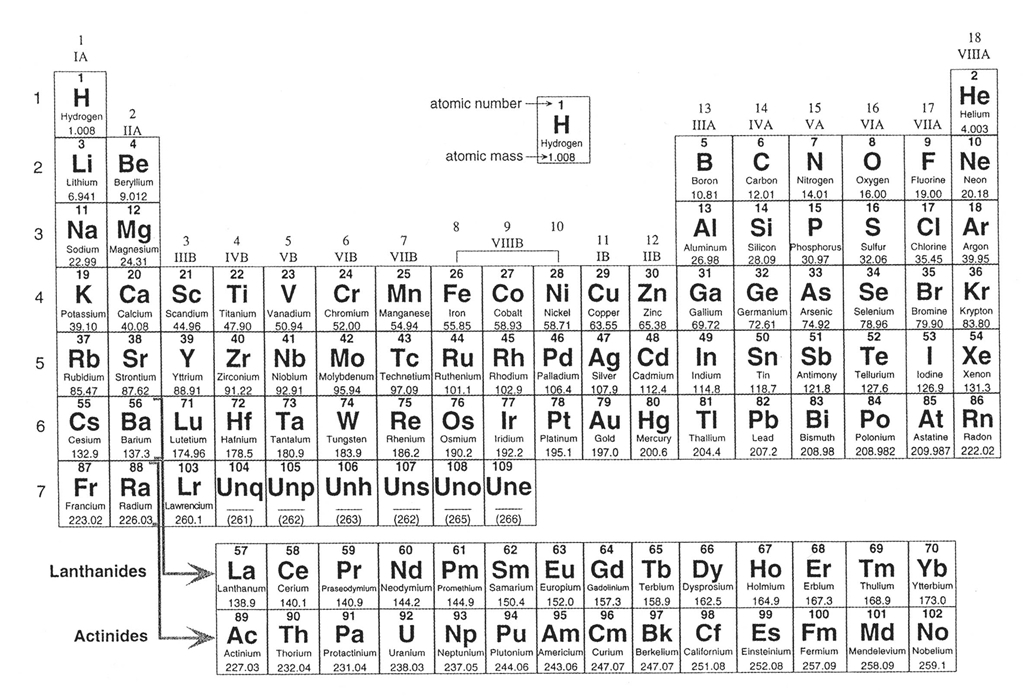
Let's begin by reviewing a little chemistry, as the 92 naturally-occurring elements are the building blocks that form more than the 5,500 different types of known minerals. These elements are organized in every science student's favorite table, the Periodic Table of the Elements (Figure 4-2), which lists the elements in order from smallest (hydrogen) to largest (uranium). The elements are arranged in order by their atomic number, essentially the number of protons in the nucleus of an atom, and each element has its own atomic number. For example, hydrogen has atomic number = 1 because it has 1 proton, helium has atomic number = 2 because it has two protons, etc. The atomic number is typically written above the large letters of the element symbol. The atomic weight of each element (the mass of all the particles in the element) is typically written below the element symbol.
|
|
|
Figure 4-2. The periodic table of the elements. Click HERE for a higher resolution PDF version. It will open in a new browser window so that you may refer to it while doing your lab. |
We will not have know all of these elements in this class, but it is important to be familiar with the most important ones. Of the 92 naturally-occurring elements, only about two dozen play a significant role in forming most of the rocks we see. In fact, Earth's Crust (by weight) is composed largely of less than 10 elements. Oxygen and silicon make up about 75% of the Crust, with lesser amounts of aluminum, iron, calcium, magnesium, sodium, potassium, etc. ). Before we look at minerals, let's review the names and symbols of some of the more important mineral-forming elements. Let's start by getting comfortable with the names and symbols for some of the elements that we will see a lot in the coming weeks.
The Quiz Me! questions A01 to A03 refer to the Periodic Table in Figure 4-2.
![]()
![]()
![]()