Part B
Geochemical Analysis
Geochemical analysis and the determination of the abundances of certain elements (like Si, Fe, Mg, etc.) and their oxides (like SiO2, FeO, MgO, etc.) can be useful in naming volcanic rocks, as well as describing the nature and origin of these rocks (e.g., melting crust or mantle) and how they originally erupted. Geochemical data is produced in various ways, but originally involves collection a fresh, representative rock sample. The composition of the whole rock or individual crystals can be analyzed. Various processing and analytical methods can then yield the relative abundances of various major elements (Si, O, Al, Fe, etc.), trace elements (Ni, Cr, Zr, etc.), and even isotopes (87Sr/86Sr, 207Pb/206Pb, etc.) that are used to describe and interpret the rock.
Major Elements
Major elements (e.g., O, Si, Al, Fe, Mg, etc.) are those that are present in the greatest relative abundance in a rock. Since most terrestrial rocks contain a large percentage of oxygen, major elements are typically grouped with oxygen and referred to as oxides (note: an oxide is simply any element combined with oxygen). The common major oxides include SiO2, Al2O3, FeO, MgO, CaO, Na2O, K2O, TiO2, P2O5, and their abundances are typically given in weight % (wt%) of the rock (e.g., 45.2 wt% SiO2). These abundances are typically measured in laboratories using techniques like X-ray fluorescence (XRF).
The percentage (by weight) of oxides like SiO2, Na2O, K2O, MgO, etc., are used to describe, classify, and interpret compositions:
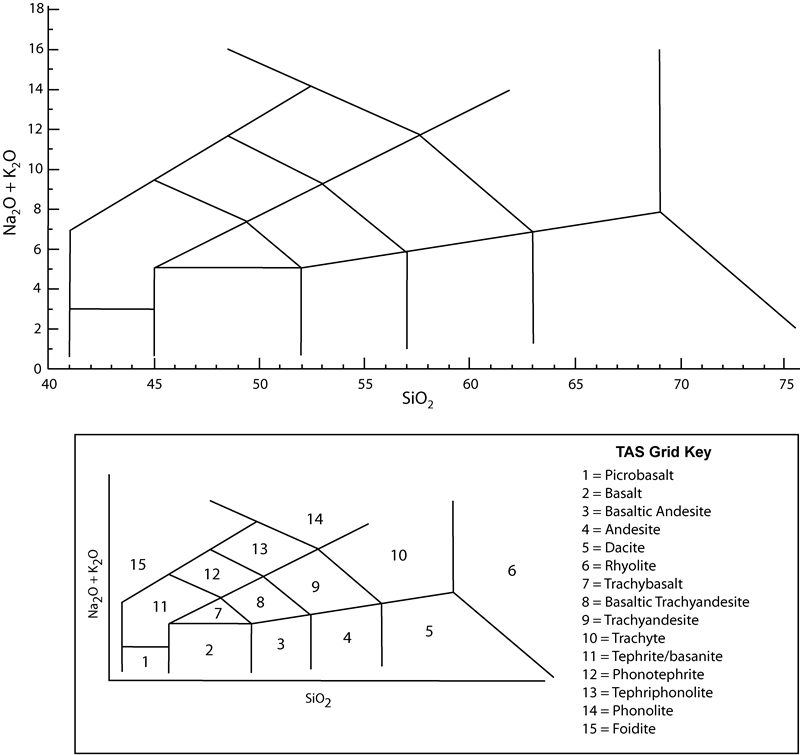
Felsic - High SiO2 (>63 wt%), Al2O3, Na2O, K2O, etc., and low MgO, FeO, and CaO.
Intermediate - SiO2 (52-63 wt%).
Mafic - Low SiO2 (45-52 wt%), Al2O3, Na2O, K2O, etc., and high MgO, FeO, and CaO.
Ultramafic - Very low SiO2 (<45 wt%) and very high MgO, FeO, and CaO.
Total Alkali - Silica (TAS) Classification
> Major element chemistry is commonly used to classify and name different types volcanic rocks. One common scheme (Le Maitre and others, 2002) compares the abundances (in weight %) of total alkalis (Na2O+K2O) versus silica (SiO2) on an X-Y diagram (note: the elements sodium and potassium are considered alkali elements). The resulting total alkali - silica (TAS) classification (see Figure 7-3) is very useful in naming and comparing volcanic rocks that may be hard to definitively classify by other means.
|
|
|
Figure 7-3. Total alkali versus silica diagram (Le Maitre and others, 2002; Le Bas and others, 1986). |
|
Example 1 |
|
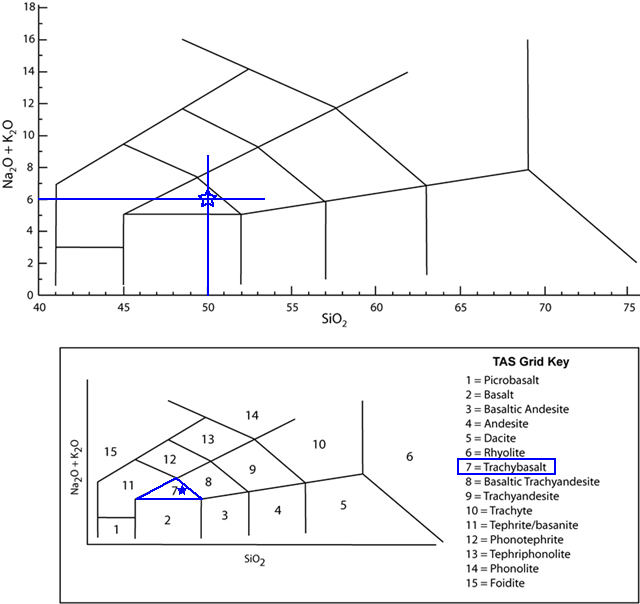
Using the TAS diagram, name the volcanic rock that has 50.0 wt% SiO2 and 6.0 wt% Na2O + K2O. |
|
Trachybasalt |
Interpreting Weight % SiO2
Proper interpretation of geochemical data can also help describe the nature and origin of a volcanic rock. For example, determining the silica content (SiO2) of a volcanic rock can be important because it relates to the origin of the magma and how the magma erupts and flows.
Origin of the Magma - In general, SiO2-rich (felsic) magmas form from the melting of continental crust, whereas SiO2-poor (mafic) magmas form from partial melting of the mantle. Intermediate magmas form from the hydrous (water-rich) partial melting of oceanic and/or continental crust, mixing of mafic and felsic magmas, etc.
How Magma Erupts and Flows - The higher the wt% SiO2, the more viscous the magma . SiO2-rich (>63 wt%) lavas are highly viscous and form short, thick flows and steep-sided lava domes. Violent eruptions of these lavas can occur because of the pressure of the trapped gases. SiO2-poor (<52 wt% SiO2) lavas are very fluid and flow for longer distances. These lavas typically erupt more passively because the gas that drives an eruption escapes more easily.
Refer to the previous discussion and the TAS diagram to answer Quiz Me! questions B21 through B30.
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()