Part D
Isotopic Age Dating
Isotopic age determinations are extremely important in piecing together the geologic histo ries of planets and moons. For example, the dark lunar lowlands are underlained mostly by basaltic lavas that erupted 3 to 4 billion years ago.How do we know this? Well, we had to go there and collect some actual rock samples first! This was one of the goals of the Apollo program. One of the first lunar samples studied was a basalt collected from Mare Tranquilitatis by Apollo 11 astronauts Neil Armstrong and Edwin "Buzz" Aldrin in 1969 (see Figure 4-15). It was isotopically dated at 3.91 ±0.01 Ga. This was just one of many rock samples collected by the six Apollo landing missions (382 kilograms of lunar surface material were collected in total) that helped us decipher the geologic history of the Moon. To better understand how "number ages" are derived, we need to briefly review some atomic basics.
|
Figure 4-15. Basalt sample 10003 collected by Apollo 11. The rock sample is about 5 cm across (left). A polarized microscope view of a thin section of sample 10003 showing the different minerals present in the basalt (right). |
|
Isotopes & Radioactive Decay
The isotopes of certain elements can be used to determine the absolute or "number" age of a mineral or rock. All 92 naturally occurring elements are composed of a dense nucleus of protons (positive electrical charge) and neutrons (no electrical charge) that is surrounded by one or more shells of electrons (negative electrical charge). Atoms of any single element (like carbon, C) always have the same number of protons (carbon has 6), but the number of neutrons may vary. Thus, several different isotopes of an element can exist, with each always having the same number of protons, but a different number of neutrons. For example, carbon-12 (12C) has 6 protons and 6 neutrons, whereas carbon-14 (14C) has 6 protons and 8 neutrons and is slightly more massive than the 12C isotope (due to the additional neutrons). Also, some isotopes will not breakdown over time (stable isotopes like 12C), whereas others will undergo radioactive decay at a rate unique for that isotope (radioactive isotopes like 14C). The decay process of radioactive isotopes has been studied for the last century or so and is used to make age determinations of rocks and minerals.
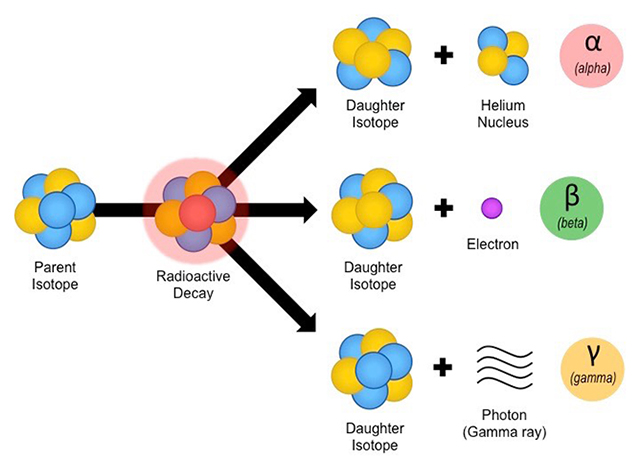
Elements like uranium, potassium, rubidium, and carbon all have radioactive isotopes (238U, 40K, 87Rb, 14C) that decay over time into other elements, eventually one that is stable. These radioactive elements are termed parent isotopes, and the resulting elements produced by different radioactive decay processes are called daughter isotopes (see Figure 4-16). For example, carbon-14 (14C) is a parent isotope that decays into a daughter isotope, nitrogen-14 (14N). There are several important parent-daughter isotope pairs:
|
238U to 206Pb |
87Rb to 87Sr |
40K to 40Ar |
14C to 14N |
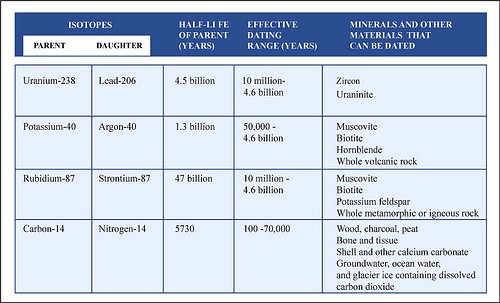
The important aspect is that the decay rate for any parent isotope is constant (we know this from direct observation), so we can use the decay process as an atomic clock to "tell time". Also, different parent isotopes decay at different rates. For instance, the time it takes for uranium-238 (238U) to decay into lead-206 (206Pb) is measured in billions of years, whereas the decay of 14C into 14N is measured in thousands of years. Thus, some isotopes (like 238U, 87Rb, 40K, etc.) are good for dating old rocks (millions or billions of years old), whereas other isotopes (like 14C) are more suitable for dating young rocks (tens of thousands of years old).
|
|
|
|
Figure 4-16. Radioactive decay of select elements can be a useful tool to date rocks and minerals. The main types of radioactive decay (left). Several important radioactive parent-daughter isotope pairs commonly used in geochronology (right). |
|
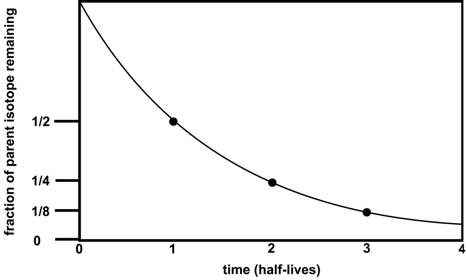
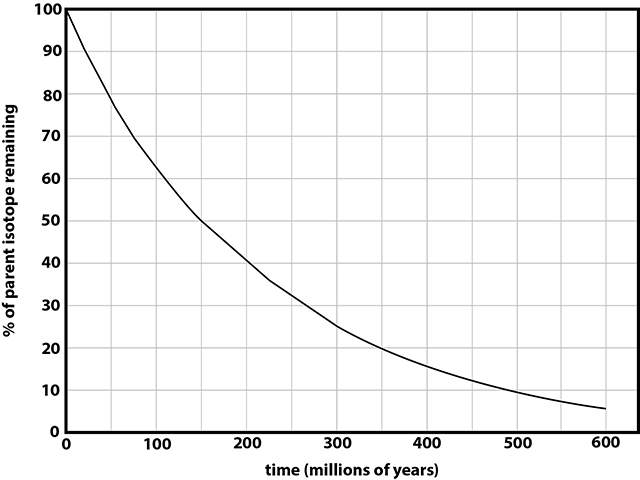
Radioactive decay rate is expressed as a unit of time called a half-life, which is the time it takes for half of the original parent isotopes to decay into daughter isotopes. After one half-life, the amount of original parent material decreases by one half (50%), after 2 half-lives, one half of one half or one quarter (25%) of the parent material remains, and so on until the parent material is exhausted. The parent isotope abundance will progressively decrease with time and the abundance of a daughter isotope will increase. This relationship is shown by the exponential curve in Figure 4-17.
|
|
|
Figure 4-17. A hypothetical isotopic decay curve. |
Only those rocks (mostly igneous rocks) that contain minerals with radioactive isotopes (like 238U, 40K, etc.) are suitable for isotopic dating. The age determined from an igneous rock usually represents its crystallization age (when it cooled from magma into a solid rock). Also, the mineral or rock must be unaltered to represent a closed system (no parent or daughter material has been added or subtracted during the decay process). Metamorphism is commonly an open-system process involving the movement of parent and/or daughter atoms, so dates from these rocks represent the age of metamorphism, not the crystallization age.
Quiz Me! questions D36 through D40 refer to the discussion above.
![]()
![]()
![]()
![]()
![]()
Determining a Rock's Absolute Age
All we really need to determine the age of the rock is the relative amount (ratio) of parent and daughter material and the half-life of the parent isotope.
|
Example 1 |
|
A sample of a basaltic lava flow is collected in the field, and sent to a laboratory, where any useful isotopes are separated and measured (using a mass spectrometer). The relative amounts of parent and daughter material from the rock (or specific minerals in the rock) are then compared to estimate how long the parent material has been undergoing radioactive decay. The rock contains 25% parent isotope X and 75% daughter isotope Y. The half-life of isotope X is 10 Ma. How old is the lava flow? |
|
There is 25% isotope X relative to 75% isotope Y. Using the decay curve in Figure 4-17, this amount (1/4) of decay must have taken 2 half-lives to occur. If the half-life for X is 10 million years, then the radioactive decay process has lasted 20 million years (2 half lives * 10 million years per half-life). 20 Ma - the lava flow cooled and crystallized 20 million years ago |
|
Example 2 |
|
A sample of a basaltic lava flow is collected in the field, and sent to a laboratory, where any useful isotopes are separated and measured (using a mass spectrometer). The relative amounts of parent and daughter material from the rock (or specific minerals in the rock) can then be compared to estimate how long the parent material has been undergoing radioactive decay. The rock contains 25% parent isotope X. How old is the lava flow? |
|
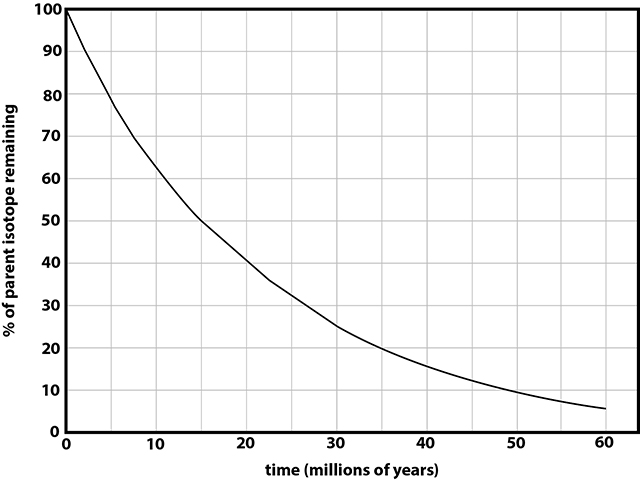
An isotopic decay curve for hypothetical isotope X. |
|
Using the isotope X decay curve above, the decay to 25% parent sotope X requires roughly 30 Ma. 30 Ma - the lava flow cooled and crystallized 30 million years ago. |
Quiz Me! questions D41 through D45 refer to Figure 4-18.
|
|
|
Figure 4-18. Isotopic decay curve for hypothetical isotope W. |
![]()
![]()
![]()
![]()
Determining a Geologic History
To most thoroughly describe the geologic history of an area, a geologist will determine the specific ages for as many rock units as possible.
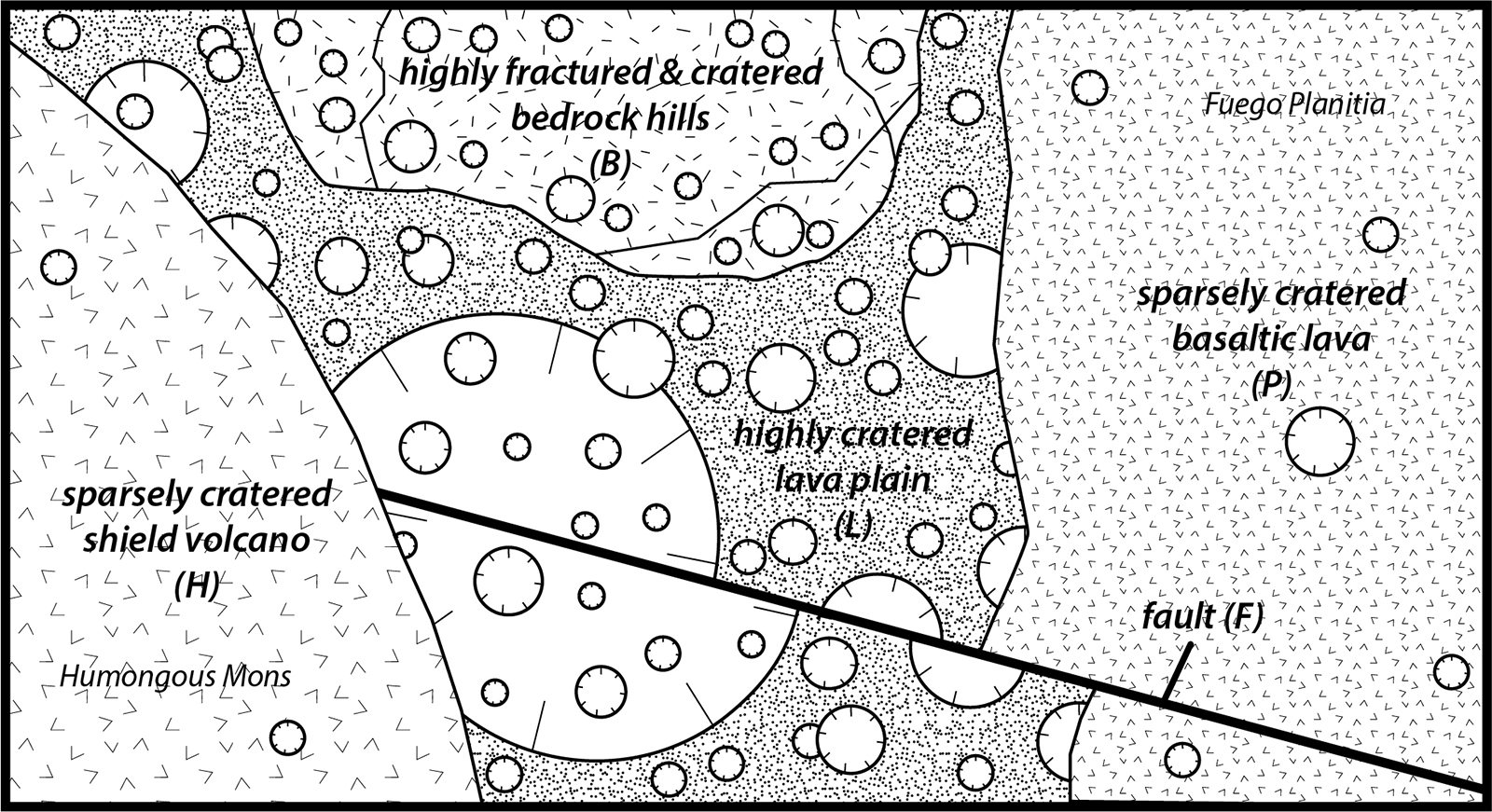
These questions refer to the decay curve in Figure 4-18 and the geologic sequence map shown in Figure 4-19.
![]()
![]()
![]()
![]()
![]()
![]()
After finishing this lesson, complete the form below: