Part A
Element & Minerals
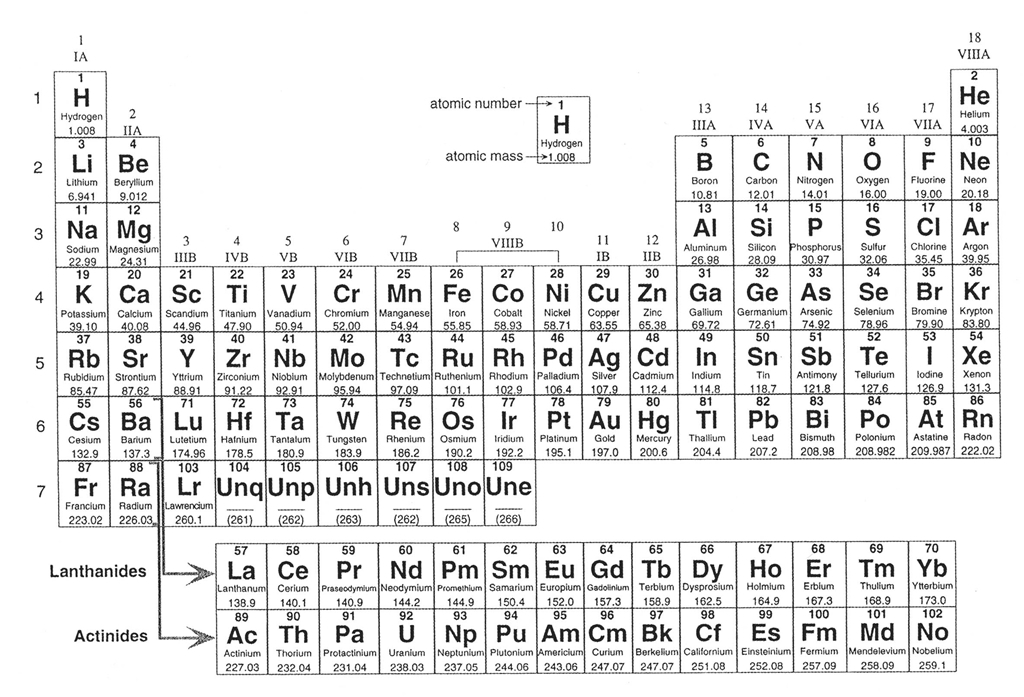
Let's begin by reviewing a little chemistry, as the 92 naturally-occurring elements are the building blocks that form more than the 5,500 different types of known minerals. These elements are organized in every science student's favorite table, the Periodic Table of the Elements (Figure 4-2), which lists the elements in order from smallest (hydrogen) to largest (uranium). The elements are arranged in order by their atomic number, essentially the number of protons in the nucleus of an atom, and each element has its own atomic number. For example, hydrogen has atomic number = 1 because it has 1 proton, helium has atomic number = 2 because it has two protons, etc. The atomic number is typically written above the large letters of the element symbol. The atomic weight of each element (the mass of all the particles in the element) is typically written below the element symbol.
|
|
|
Figure 4-2. The periodic table of the elements. Click HERE for a higher resolution PDF version. It will open in a new browser window so that you may refer to it while doing your lab. |
We will not have know all of these elements in this class, but it is important to be familiar with the most important ones. Of the 92 naturally-occurring elements, only about two dozen play a significant role in forming most of the rocks we see. In fact, Earth's Crust (by weight) is composed largely of less than 10 elements. Oxygen and silicon make up about 75% of the Crust, with lesser amounts of aluminum, iron, calcium, magnesium, sodium, potassium, etc. ). Before we look at minerals, let's review the names and symbols of some of the more important mineral-forming elements. Let's start by getting comfortable with the names and symbols for some of the elements that we will see a lot in the coming weeks.
The Quiz Me! questions A01 to A03 refer to the Periodic Table in Figure 4-2.
![]()
![]()
![]()
What is a Mineral?
What is a mineral, you ask? Well, a mineral is a naturally-occurring, inorganic substance with crystalline structure, a definite chemical composition, and diagnostic physical properties. So, what does that mean?
|
Figure 4-3. Minerals and non-minerals. Clockwise from top left: solid water ice (a mineral), liquid water (not a mineral - no crystalline structure), plastic (not a mineral - man-made, no crystalline structure, organic), and pyrite (a mineral). |
|
A mineral is defined as:
Naturally-occurring - You can find it in nature. Not man-made or synthetic. It should be noted that materials having characteristics very similar to those of real minerals can be manufactured in factories, but are considered synthetic minerals and are not true minerals in a geologic sense.
Inorganic - Not alive or composed of organic (carbon-based) matter. Almost all minerals are inorganic except for the rare instances where geological processes can form organic substances that can be considered minerals.
Crystalline structure - An orderly arrangement of atoms in a substance. Materials having crystalline structure can be called crystals.
Definite chemical composition - The same combination of elements (e.g., galena is always PbS, not P2S or PbS2). Slight variations can occur with secondary elements, but the main composition is always the same.
Diagnostic physical properties - A diverse suite of characteristics displayed by a substance (e.g., color, shape, hardness, etc.) that can be reliably used to identify the mineral.
Common Rock-forming Minerals
Only a small fraction of the over 5,500 known minerals are of major importance in most rock-forming minerals. There are several important rock-forming mineral groups, including silicates, carbonates, and others (like oxides, sulfides, sulfates, etc.), each with many different varieties of similar minerals. For instance, the mineral hornblende is a common, double-chain silicate mineral (inosilicate) and is an important member of the amphibole group. But only a few dozen rock-forming minerals compose most of the common rock types, and in this class, we will typically focus on the more common mineral representatives. As you progress deeper into geology, you will become familiar with many of the lesser known minerals.
Silicate minerals are composed of various arrangements of silicon (Si) and oxygen (O), and other elements. These are the most important rock-forming minerals and commonly (but not always) form at high temperatures from magmas or under various metamorphic environments within the Earth (see Figure 4-4). Carbonate minerals are composed of carbon (C), oxygen (O), and other elements, and are also an important rock-forming mineral group (see Figure 4-4). There are several other important groups of minerals, including sulfides, sulfates, oxides, etc.
Differences in mineral chemical composition and atomic structure give each mineral its physical properties, which then allow a mineral to be identified and classified. Color is an initial indication of the chemistry of a silicate mineral: light-colored minerals are richer in silica (SiO2) and also potassium (K), sodium (Na), and aluminum (Al), whereas the dark-colored minerals have less silica and are richer in iron (Fe), magnesium (Mg), and calcium (Ca). The shape of a mineral crystal, either by its crystal form or by how it breaks, can yield clues to its atomic structure. Let's look at some brief descriptions of the more common rock-forming minerals.
|
Figure 4-4. Igneous and sedimentary minerals. At left, silvery muscovite, with its one cleavage plane, forms muscovite "books" in this pegmatite dike along the Hermit Trail in Grand Canyon NP. At right, large calcite crystals growing in a limestone opening (~1 m wide) along the Colorado River (mile 34.8). |
|
Mineral Descriptions
![]() Olivine / (Mg,Fe)2SiO4 / Granular or grainy appearance; pale to dark green glassy grains; looks like green sand or green sugar. H = 7. No obvious cleavage, but has conchoidal fracture. Common in mafic and ultramafic igneous rocks.
Olivine / (Mg,Fe)2SiO4 / Granular or grainy appearance; pale to dark green glassy grains; looks like green sand or green sugar. H = 7. No obvious cleavage, but has conchoidal fracture. Common in mafic and ultramafic igneous rocks.
![]() Amphibole (Hornblende) / Ca2(Fe,Mg)4Al(Si7Al)O22(OH)2 / Elongated crystals that are typically black or greenish-black color. H = 5.5. Two cleavages form corners not at right angles (56º or 124º angles), so it looks diamond-like in cross section. Common in many igneous rocks, as well as some metamorphic rocks.
Amphibole (Hornblende) / Ca2(Fe,Mg)4Al(Si7Al)O22(OH)2 / Elongated crystals that are typically black or greenish-black color. H = 5.5. Two cleavages form corners not at right angles (56º or 124º angles), so it looks diamond-like in cross section. Common in many igneous rocks, as well as some metamorphic rocks.
![]() Pyroxene (Augite) / (Mg,Fe)SiO3 / Prism- or blocky-shaped crystals that are usually dark army green or black in color. Two cleavages intersect at nearly right angles (~90º). It may look octagonal in cross section. H = 5.5. Common in mafic and ultramafic igneous rocks.
Pyroxene (Augite) / (Mg,Fe)SiO3 / Prism- or blocky-shaped crystals that are usually dark army green or black in color. Two cleavages intersect at nearly right angles (~90º). It may look octagonal in cross section. H = 5.5. Common in mafic and ultramafic igneous rocks.
![]() Plagioclase feldspar / NaAlSi3O8 to CaAl2Si2O8 / A series of Na- to Ca-rich feldspars. Commonly tabular in shape with white to gray color. May have parallel "grooves" (striations) on the flat surfaces; Two cleavages intersects at nearly right angles (~90º). H = 6. Common in many igneous, metamorphic rocks, and clastic sedimentary rocks.
Plagioclase feldspar / NaAlSi3O8 to CaAl2Si2O8 / A series of Na- to Ca-rich feldspars. Commonly tabular in shape with white to gray color. May have parallel "grooves" (striations) on the flat surfaces; Two cleavages intersects at nearly right angles (~90º). H = 6. Common in many igneous, metamorphic rocks, and clastic sedimentary rocks.
![]() Alkali feldspar (Orthoclase) / NaAlSi3O8 to KAlSi3O8 / Tabular or blocky crystals that are white to pinkish color. May contain wavy colored lines of Na-felspar (exsolution lamellae).Two cleavages intersects at nearly right angles (~90º). H = 6. Common in many felsic igneous rocks and metamorphic rocks.
Alkali feldspar (Orthoclase) / NaAlSi3O8 to KAlSi3O8 / Tabular or blocky crystals that are white to pinkish color. May contain wavy colored lines of Na-felspar (exsolution lamellae).Two cleavages intersects at nearly right angles (~90º). H = 6. Common in many felsic igneous rocks and metamorphic rocks.
![]() Biotite / K(Mg,Fe)3(AlSi3O10)(OH)2 / Flaky and breaks into thin flexible sheets. Dark color, usually black to brownish-black. One perfect (basal) cleavage. Gray-brown streak. H = 3-2.5. Common in many felsic to intermediate igneous rocks and metamorphic rocks.
Biotite / K(Mg,Fe)3(AlSi3O10)(OH)2 / Flaky and breaks into thin flexible sheets. Dark color, usually black to brownish-black. One perfect (basal) cleavage. Gray-brown streak. H = 3-2.5. Common in many felsic to intermediate igneous rocks and metamorphic rocks.
![]() Muscovite / KAl2(AlSi3O10)(OH)2 / Basal cleavage allows this mineral to break into thin, flexible sheets that individually may be transparen Usually pale brown to silver in color. H = 2.5-2. Common in many felsic igneous rocks and metamorphic rocks.
Muscovite / KAl2(AlSi3O10)(OH)2 / Basal cleavage allows this mineral to break into thin, flexible sheets that individually may be transparen Usually pale brown to silver in color. H = 2.5-2. Common in many felsic igneous rocks and metamorphic rocks.
![]() Quartz / SiO2 / Often irregular in shape, but can form hexagonal prism crystals. Depending on impurities, can have many colors. No cleavage, but has conchoidal fracture. H = 7. Common in many felsic igneous rocks, metamorphic rocks, and sedimentary rocks.
Quartz / SiO2 / Often irregular in shape, but can form hexagonal prism crystals. Depending on impurities, can have many colors. No cleavage, but has conchoidal fracture. H = 7. Common in many felsic igneous rocks, metamorphic rocks, and sedimentary rocks.
![]() Calcite / CaCO3 / Can have many forms, but cleavage causes it to break into rhombic shapes. Typically light-colored with many color varieties. Reacts with acid. H = 3. May display double refraction and fluorescence. Common in chemical sedimentary rocks.
Calcite / CaCO3 / Can have many forms, but cleavage causes it to break into rhombic shapes. Typically light-colored with many color varieties. Reacts with acid. H = 3. May display double refraction and fluorescence. Common in chemical sedimentary rocks.
![]() Gypsum / CaSO4 * 2H2O / Tabular shape or fibrous. Commonly clear or white colored. Two cleavages that intersect not at 90º (66º or 114º angles) form prismatic sheets. H = 2. An evaporite mineral common in sedimentary rocks.
Gypsum / CaSO4 * 2H2O / Tabular shape or fibrous. Commonly clear or white colored. Two cleavages that intersect not at 90º (66º or 114º angles) form prismatic sheets. H = 2. An evaporite mineral common in sedimentary rocks.
![]() Halite / NaCl / Forms cubic crystals. Typically light-colored with many color varieties. Three cleavages intersect at 90 to form cubes. H = 3. An evaporite mineral common in sedimentary rocks.
Halite / NaCl / Forms cubic crystals. Typically light-colored with many color varieties. Three cleavages intersect at 90 to form cubes. H = 3. An evaporite mineral common in sedimentary rocks.
![]() Pyrite / FeS2 / Metallic luster. Brassy yellow color that can tarnish brown. Often massive or forms cubes. Dark gray streak. H = 6.5-6. Specific gravity = 5.0. Commonly known as "fools gold".
Pyrite / FeS2 / Metallic luster. Brassy yellow color that can tarnish brown. Often massive or forms cubes. Dark gray streak. H = 6.5-6. Specific gravity = 5.0. Commonly known as "fools gold".
![]() Hematite / Fe2O3 / Metallic to non-metallic luster. Metallic gray to earthy red color. Red to red-brown streak. H = 5.5. Specific gravity = 5.3-4.9. A common component in the cement of clastic sedimentary rocks.
Hematite / Fe2O3 / Metallic to non-metallic luster. Metallic gray to earthy red color. Red to red-brown streak. H = 5.5. Specific gravity = 5.3-4.9. A common component in the cement of clastic sedimentary rocks.
![]() Garnet / (Mg,Fe,Mn,Ca)3Al2Si3O12 / Commonly forms dodecahedral (12-sided) to cubic crystals. Can have many colors, but typically reddish-brown. No cleavage. H = 7. Common in metamorphic rocks and felsic igneous rocks.
Garnet / (Mg,Fe,Mn,Ca)3Al2Si3O12 / Commonly forms dodecahedral (12-sided) to cubic crystals. Can have many colors, but typically reddish-brown. No cleavage. H = 7. Common in metamorphic rocks and felsic igneous rocks.
Quiz Me! questions A04 to A10 refer to the mineral descriptions above.
![]()
![]()
![]()
![]()
![]()
![]()
![]()